noble gas configuration calculator|noble gas configuration worksheet answers : Manila Learn how to write a noble gas configuration for any atom using the Aufbau principle and the periodic table. See examples and a list of noble gas configurations for . WARNING What youre about to see is nearly uncut fooge of Daisys Destruction, #warning #youre #nearly #uncut #fooge #daisys #destruction. 12 comment. The fuck is Daisy's Destruction? Kickerofnoon 2 jan 2021. 0 2. What is this some kinda princess daisy shit? Like from mario? Kreamie 1 jan 2021. 1 2. Too boring, couldnt spell .
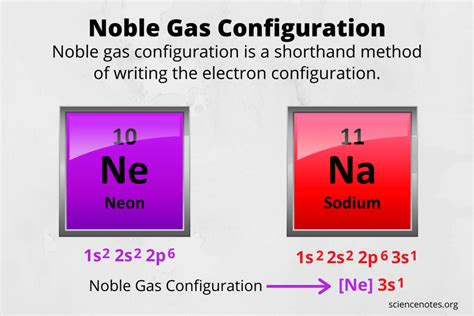
noble gas configuration calculator,Noble Gas Configuration Calculator is an online tool that will help you find the noble gas electron configuration of any element in a second.
Find the electron configuration of any element using this tool. Learn the rules, notation, and examples of electron configuration and valence electrons.
Find the electron configuration of any element using this online tool. Learn the rules, shells, subshells, and atomic number of each element with examples and F.Enter an element's name or symbol to see its noble gas electron configuration. Send feedback | Visit Wolfram|Alpha. Get the free "Electron Configuration" widget for your .
Learn how to write a noble gas configuration for any atom using the Aufbau principle and the periodic table. See examples and a list of noble gas configurations for .A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons.Learn how to write noble gas shorthand electron configurations for elements using the periodic table. See examples, videos, questions and answers on noble gas shorthand .

What would the noble gas electron configuration for an element look like if the element is a noble gas? Would it be. [Ar] or. [Ne]3s^23p^6 ? •. ( 16 votes) The noble gas configuration is written as the elemental symbol of the noble gas in the period before the element followed by .noble gas configuration calculator noble gas configuration worksheet answersOxygen, for example, has the electron configuration 1s 2 2s 2 2p 4, whereas the oxygen anion has the electron configuration of the noble gas neon (Ne), 1s 2 2s 2 2p 6. The two additional electrons required to fill .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .noble gas configuration worksheet answersSo that's the electron configuration for silicon. Now, we can write it out using noble gas notation. And compare, so, the noble gas immediately preceding silicon, if we go up a row and then move over, we see that it's neon. So we write neon in brackets. And then, the other electrons are the ones that come after neon.
They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration. The noble gas configuration is written as the elemental symbol of the noble gas in the period before the element followed by the element’s remaining electrons. For instance, sodium’s full configuration is 1s 2s 2 2 2p 6 3s 1 and neon’s is 1s 2s 2 2 2p 6. So, sodium’s noble gas configuration is [Ne]3s 1 . Part 1.
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.To use the Electronic Configuration Calculator you must follow these steps: Select the chemical element. Indicate if you want to obtain the electronic configuration of the selected element or of an ion. In case of selecting ion, you must indicate its charge, using the + or – signs followed by the number; For example, if we want to obtain the .A Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already electron .
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/science/chemistry/electronic-struct. Noble Gas Configuration. FlexBooks 2.0 >. CK-12 Chemistry for High School >. Noble Gas Configuration. Written by: Ck12 Science. Fact-checked by: The CK-12 Editorial Team. Last Modified: Jan 02, 2023.So one notation folks often use is noble gas configuration where instead of saying, okay, this is carbon, they could say that, hey look, carbon is going to have the electron configuration of helium, remember, the noble gasses are these Group 8 elements right over here, so it's going to have the electron configuration of helium which tells us .Noble Gas Configuration Calculator is an online tool that will help you find the noble gas electron configuration of any element in a second. Note: You can bookmark this page or share. Read More Noble Gas Configuration CalculatorThe noble gases (historically the inert gases, sometimes referred to as aerogens) . As a result of a full shell, the noble gases can be used in conjunction with the electron configuration notation to form the noble gas notation. To do this, the nearest noble gas that precedes the element in question is written first, and then the electron .To write the electron configuration of nitrogen using a shorthand method, we start with its atomic number, which is 7. Using an electron configuration calculator or chart, we find that the full electron configuration is 1s² 2s² 2p³. Now, for the shorthand method, we locate the last noble gas before nitrogen, which is helium (He).
Noble Gas Configurations. Sodium, element number eleven, is the first element in the third period of the periodic table. Its electron configuration is 1s 2 2s 2 2p 6 3s 1. The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the . This chemistry video explains how to write the electron configuration of an element using noble gas notation.Speed of Light, Frequency, Wavelength: http. Condensed electron configuration calculator. To help all those students or professionals related to the field of chemistry we have created the Electronic Configuration Calculator. Natural gas royalty calculator. տնօրեն 374-285-53712 374-91-208325. To write the electron configuration which is unique to each element on the periodic table .
noble gas configuration calculator The sodium ions are sodium atoms which have lost an electron, giving them the structure 1 s2 2 s2 2 p6, the same as that of the noble-gas neon. All electrons in both kinds of ions are paired. This page titled 6.5: Ions and Noble-Gas Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated .

Atoms and atomic ions with sequences of completely filled electron shells exhibit enhanced stability. The prime examples are the noble gases, He, Ne, Ar, Kr, Xe, and Rn, containing one of the "magic numbers" of electrons: 2, 10, 18, 36, 54, and 86, respectively. These gases are colorless, odorless, and chemically inert (although a few .
noble gas configuration calculator|noble gas configuration worksheet answers
PH0 · shorthand electron configuration calculator
PH1 · noble gas electron configuration example
PH2 · noble gas configuration worksheet answers
PH3 · noble gas configuration worksheet
PH4 · noble gas configuration for sodium
PH5 · noble gas configuration for aluminum
PH6 · noble gas configuration chart
PH7 · ground state electron configuration calculator
PH8 · Iba pa